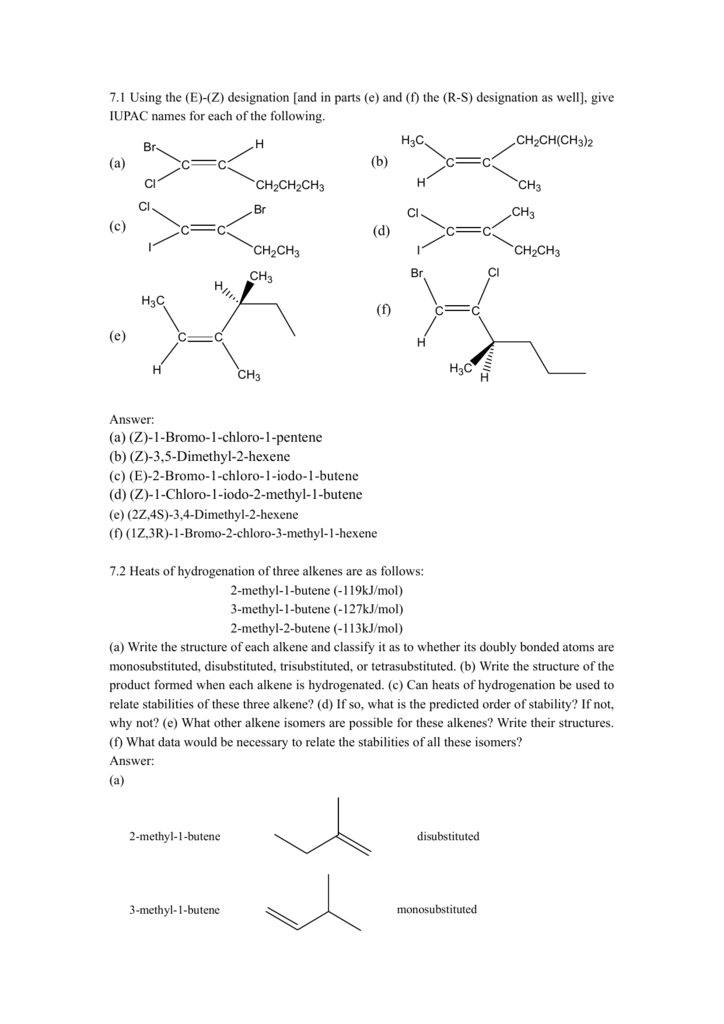
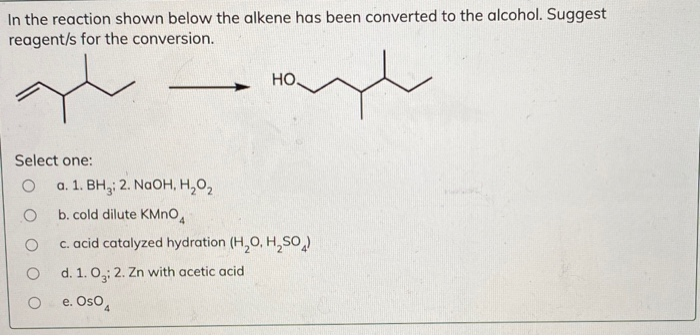
A) H 2 O, concentrated H 2 SO 4 B) Br 2, H 2 O C) BH 3, THF;CH 3 —CH(Cl)CH 2 CH 3 will be more easily hydrolysed because it will form secondary carbocation which is more stable than primary carbocation Question 75 Answer 4Bromo3methylpent2ene Question 76 Draw the structure of the following compound 4 Bromo3methylpent2ene Answer Short Answer Type Questions II 3 Marks Question 77Showing 130 of 334 results for "2methylpent2ene" Advanced Search Structure Search Relevance Compare 4Hydroxy4methylpent2ynoic acid 4Hydroxy4methylpent2ynoic acid CAS Number (E)BUT2ENE2,3DIYLDIBENZENE AND (Z)BUT2ENE2,3DIYLDIBENZENE Molecular Weight Linear Formula C 32 H 32 Product
2 Chloro 4 Methyl Pent 2 Ene C6h11cl Pubchem
E-2-chloro-3-methylpent-2-ene
E-2-chloro-3-methylpent-2-ene-See the answer Which of the following alkenes is the major product when2bromo2methylpentane is treated with sodium ethoxide in ethanol?a 4methylpent1ene b (Z)4methylpent2ene c (E)4methylpent2ene d 2methylpent2ene e2methylpent1ene Dehydrohalogenation of 2bromobutane in the presence of astrong base proceeds via whichA) 2methylpentane b) 1,2dibromo2methylpentane c) 1bromo2methylpentane d) 2bromo2methylpentane e) 4bromo2methylpentane f) none of the above 2 When you react 2methylbutane with Br2 and uv light, the major product will be




Draw The Bond Line Structures Of The Following Compound Whose Iupac Names Are Given As Under A 2 Bromobutane B 1 Chloro 3 Methylbutane C 2 Bromo 2 Methylpropane D 4 Chloro 4 Methylpent 2 Ene E
With cyclohexanone(E)1chloro3methylpent2 to form 2(Z)3methylpent2enylThe LibreTexts libraries are Powered by MindTouch ® and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot We also acknowledge previous National Science Foundation support under grant numbers ,Find (E)2(hydroxymethyl)3methylpent2enal and related products for scientific research at MilliporeSigma
IUPAC name 4bromo4methylpent2ene Question 21 What happens when ethyl chloride is treated with aqueous KOH?A 1 mole H2, Ni and heat b 2 moles H2, Ni and heat c 2 moles of Cl2 in CCl4 d 1 mole of Br2 in CCl4 c 2Bromohex1ene d E2Bromohex2ene e A mixture of E and Z isomers of 1bromohex1eneThe proper name for this molecule is either trans2fluoro3methylpent2ene because the alkyl groups that form the backbone chain (ie, methyl and ethyl) reside across the double bond from each other, or (Z)2fluoro3methylpent2ene because the highestpriority groups on each side of the double bond are on the same side of the double bond
2Methyl2butene, 2m2b, 2methylbut2ene, also amylene is an alkene hydrocarbon with the molecular formula C 5 H 10 Used as a free radical scavenger in trichloromethane (chloroform) and dichloromethane (methylene chloride) John Snow, the English physician, experimented with it in the 1840s as an anesthetic, but stopped using it for unknown reasons See alsoFrom the Markownikoff rule 2chloro1iodopropane should be the principal product because chlorine is more electronegative than iodine, so think of it as the addition of I δ Cl δOChlorotoluene 1Chloro2methylbenzene or 2Chlorotoluene Benzyl chloride Chlorophenylmethane Table 101 Common and IUPAC Names of some Halides 4Bromo3methylpent2ene (iv) 1Bromo2methylbut2ene (v) 1Bromobut2ene (vi) 3Bromo2methylpropene Example 102 SolutionSolutionSolution ± Chemistry 294



Final Exam Answer Key
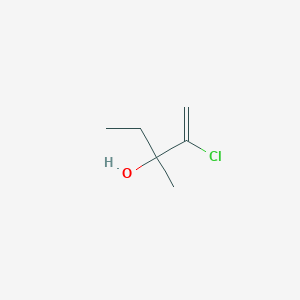



2 Chloro 3 Methyl Pent 1 En 3 Ol C6h11clo Pubchem
C6H12, (E)3Methyl2pentene, Molecules Containing Five or More Carbon Atoms DOI /_447 Study of the composition of gas from the catalytic cracking of vacuum gas oil, by means of capillary gas chromatography, Chemistry and Technology of Fuels and OilsQuestion (2R,3S)2chloro3methylpentane Reacts With Sodium Methoxide In Methanol To Give (Z)3 Methylpent2ene As The Major Product And (E)3methylpent2ene As The Minor Product In The Space Below A) Write The Structures Of Both Products B) Write A General Mechanism That Shows How Both Products Were Formed Using Curly Arrows To Show ElectronNa, NH3 Which of the following reagents should be used to convert hex3yne to (Z)hex3ene?




Nomenclature Introduction Of Major Families Of Organic Compounds Ppt Download



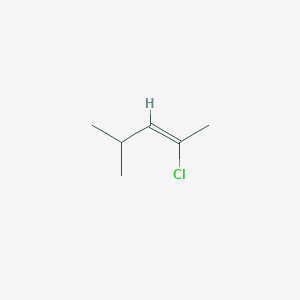
E 4 Chloro 3 Methyl Pent 2 Ene Chemsink
Bioaccumulation Estimates from Log Kow (BCFWIN v217) Log BCF from regressionbased method = 31 (BCF = 1074) log Kow used 355 (estimated) Volatilization from Water Henry LC 0663 atmm3/mole (estimated by Bond SAR Method) HalfLife from Model River 1012 hours HalfLife from Model Lake 9413 hours (3922 days) Removal In WastewaterThe compound is (E)but2ene A minor addition to the rule to allow for isotopes of, for example, hydrogen Deuterium is an isotope of hydrogen having a relative atomic mass of 2 It still has only 1 proton, and so still has an atomic number of 1 However, it isn't the same as an atom of "ordinary" hydrogen, and so these two compounds areShowing 130 of 1 results for "3methylpent2ene" Advanced Search Structure Search Relevance Compare 3Chlorobicyclo321oct2ene, mixture of endo and exo 3(6CHLORO2PYRIDINYL)8METHYL8AZABICYCLO(321)OCT2ENE 2BUTENEDIOATE 3(6CHLORO2PYRIDINYL)8METHYL8AZABICYCLO(321)OCT2ENE 2BUTENEDIOATE Molecular




3 Chloro 2 Methylpent 2 Ene 71 6 Wiki
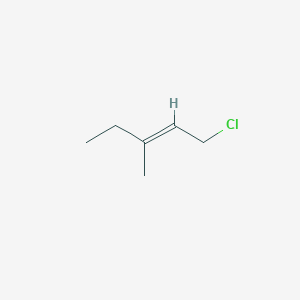



E 1 Chloro 3 Methylpent 2 Ene C6h11cl Pubchem
InChI=1/C6H11Cl/c146 (7)5 (2)3/h45H,13H3/b64 Molecular Formula C6H11Cl Reactions where this compound is a product ( Cross Metathesis) 0 from prop1ene, 2chloro3methylbut1ene from 2chloro3methylbut1ene, prop1eneIUPAC name Propane1,2,3triol 23 Write the IUPAC name of the following compounds Solution (A) IUPAC name of the compound is 3Ethyl5methyl hexane2,4diol (B) IUPAC name of the compound is1Methoxy3nitrocyclohexane 24 Write the IUPAC name of the compound given below Solution IUPAC name of the compound is 3Methylpent2ene1,21 When 2methylpent1ene is reacted with HBr containing peroxide, what will be the name of the resulting compound?



Z 1 Bromo 1 Chloro 1




E 3 Chloro 4 Methylpent 2 Ene C6h11cl Pubchem
E is a British owned energy supplier based in Birmingham We ensure everything is simple and straightforward for our customers Our focus is on keeping costs down so you pay less E is all about saving money Switch to us We ensure everything is(1) H Н CH2CH3 H н CH3 (2) Н CH2CH3 H Н (3) CH32 moles H2, Ni and heat Which of the following reagents should be used to convert hex3yne to (E)hex3ene?



Final Exam Answer Key




Write Structures Of The Following Compounds I 2 Chloro 3 Methylpentane Ii 1 Chloro 4 Ethylc Youtube
Check important questions and answers for Class 12 Chemistry Board Exam from Chapter 10 Haloalkanes and Haloarenes These questions are based on the latest CBSE Class 12 Chemistry Syllabus Chemsrc provides 1chloro3methylpent2ene(CAS#) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc Articles of 1chloro3methylpent2ene are included as wellLower right example methylpent2ene and Z3methylpent2ene (repeated further down with skeletal formulae) To understand the two lower left and right examples apply the Priority Rules to alkenes for E/Z ('geometrical') isomerism For each carbon of the double bond the higher priority atom/group is worked out



2
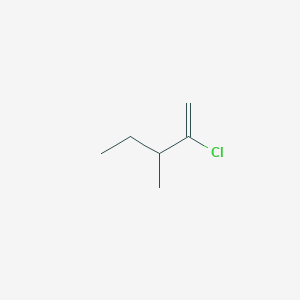



2 Chloro 3 Methylpent 1 Ene C6h11cl Pubchem
Followed by H 2 O 2 / OH D) Hg(OAc) 2, THFH 2 OWith butan2one(E)1chloro3methylpent2 to form (E)3,6dimethyloct5en2one;Which of the following reagents will convert 1 mole of 3methylpent1yne into 3methylpentane?



Ass 1aa3 Org




3 Chloro 2 Methyl Pent 2 Enal 1679 39 6 Wiki
› cis 4 methylpent 2 ene Filter by All Education Study Learning Search 4Methyl1pentene C6H12 PubChem › See more all of the best education on wwwnihgov Education Laboratory Chemical Safety Summary (LCSS) Datasheet Molecular Formula C6H12 Synonyms 4METHYL1PENTENEOf these is 2chloro3methylpentane Identify the other product A) 1Chloro3methylpentane B) 3Chloro3methylpentane Which reaction conditions would you select to synthesize 3methylpentan2ol from 3methylpent2ene?(1) CH3 (2) CH (3) CH (4) CH 2 Which of the following compounds exists as a geometric isomer?




Organic Chemistry Alkenes




Chapter 7 Key Answers Pdf Alkene Hydrogenation
212 General formula notes on the various associated unsaturated series of hydrocarbons and the many styles of representing the molecular formula and structure of alkenes The open chain alkenes with one 'ene' group have the general formula C n H 2n (n = 2, 3, 4 etc), they are isomeric with cycloalkanes from C 3 onwards n must be >1 to give a C=C double bond3Chloro2methyl2pentene ACD/IUPAC Name 3Chloro2méthyl2pentène French ACD/IUPAC Name 3chloro2methylpe nt2ene RN pent2ene, 3chlor o2methylPredicted ACD/Labs;The (E)1chloro3methylpent2ene molecule consists of 11 Hydrogen atom(s), 6 Carbon atom(s) and 1 Chlorine atom(s) a total of 18 atom(s) The molecular weight of (E)1chloro3methylpent2ene is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be



2



5 Chloro 2 Methylpent 2 Ene Get Quote
(Delhi 13) Answer Question 22 Write the IUPAC name of (CH 3) 2 CHCH(Cl)CH 3 (Delhi 13) Answer IUPAC name 2chloro3methylbutane Question 23 Which compound in the following pair undergoes faster S N 1 reaction (DelhiName the following compound according to the IUPAC rules CH3 CH2CH3 H CH3 (1) cis3methylpent2ene;Find 4methylpent3en2one and related products for scientific research at MilliporeSigma




2 Chloro 3 Methyl 1 Penten 3 Ol C6h11clo Density Melting Point Boiling Point Structural Formula Synthesis




2 Pentene 1 Chloro 3 Methyl Z 3 Wiki
Transcribed image text QUESTION 6 Name the following compound Br Br A (S,E)2,4dibrorho3methylpent3ene B (R,E)2,4dibromo3methylpent3ene C (R,Z)24dibromo3methylpent2ene D (S,E)2,4dibromo3methylpent2ene E (R, E)2,4dibromo3methylpent2ene QUESTION 7 What is the correct IUPAC name for the following compound?Question 1 Which of the following is the correct structure of 3chloro5methylcyclohexene?Eg CH 3 CH=CH 2 ICl ===> CH 3 CHICH 2 Cl or CH 3 CHClCH 2 I ;




Which Of The Following Is The Major Product In The Electrophilic Addition Of Hcl To 2 Methylpent 2 Ene Homeworklib



Final Exam Answer Key
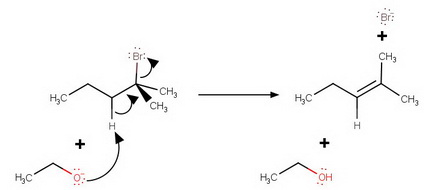
The simple mechanism shown for addition of HBr to but2ene applies to a large number of electrophilic additions We can use this mechanism to predict the outcome of some fairly complicated reactions For example, the addition of HBr to 2methylbut2ene could lead to either of two products, yet only one is observed not observed C CH H Br CH 3Which of the following reagents will convert 1 mole of 3methylpent1yne into 3methylpentane?Home Tables for Chemistry Compound classes Structure and physical data for Home 🙚 Tables for Chemistry 🙘 Compound classes 🙘
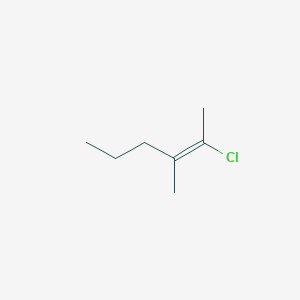



E 2 Chloro 3 Methylhex 2 Ene C7h13cl Pubchem
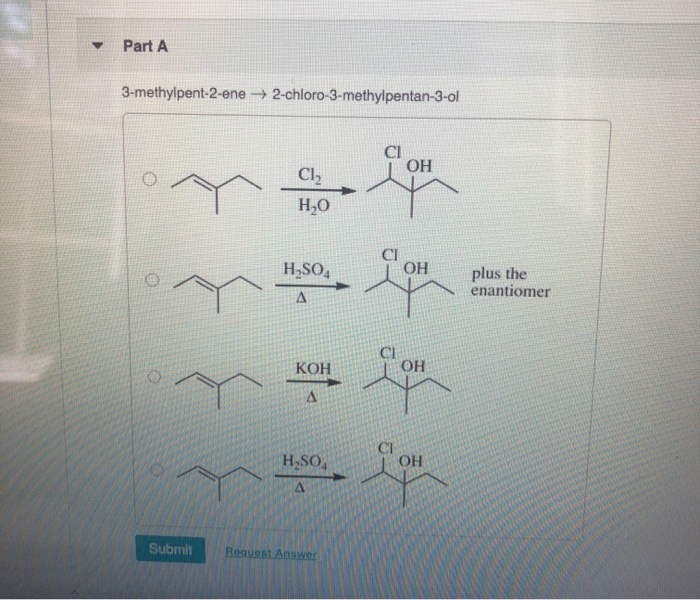



R Part A 3 Methylpent 2 Ene Chegg Com
(E)4Bromo1chloro3methylpent2ene C6H10BrCl CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and morePredicted data is generated using the ACD/Labs Percepta Platform PhysChem Module Density 09±01 g/cm 3 Boiling PointA) b) c) 10 (15 points) In the presence of acid such as H2SO4, 3,4dimethyl1pentene undergoes isomerization to 2,3dimethyl2pentene Write a complete mechanism, showing all steps and using electronpushing arrows 11 (15 points) Predict the products that would result from free radical monochlorination of 1,1dimethylcyclobutane



3 Chloro Pent 2 Ene Molbase
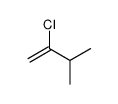



2 Chloro 3 Methylbut 1 Ene Cas 64 7 Chemsrc
Systematic names 1,2dihydroxybenzene Synonyms AIBN Trade names Aspirin Registry numbers SMILES O=C(OCC)C InChl InChI=1/CH4/h1H4 Search Search Hits Limit Filter Single/Multicomponent Search Any Search SingleComponent Structures Only58) The compound produced when 3methylpent2ene undergoes hydrogenation in the presence of a platinum catalyst is _____ 3methylpentane 59) The trans isomers of cycloalkenes with rings containing fewer than __________ atoms are unstable at room temperature



3 Chloro Pent 2 Ene Molbase




Which Structure Represents 2 Chloro 3 Methyl But 2 En 1 Ol Brainly In
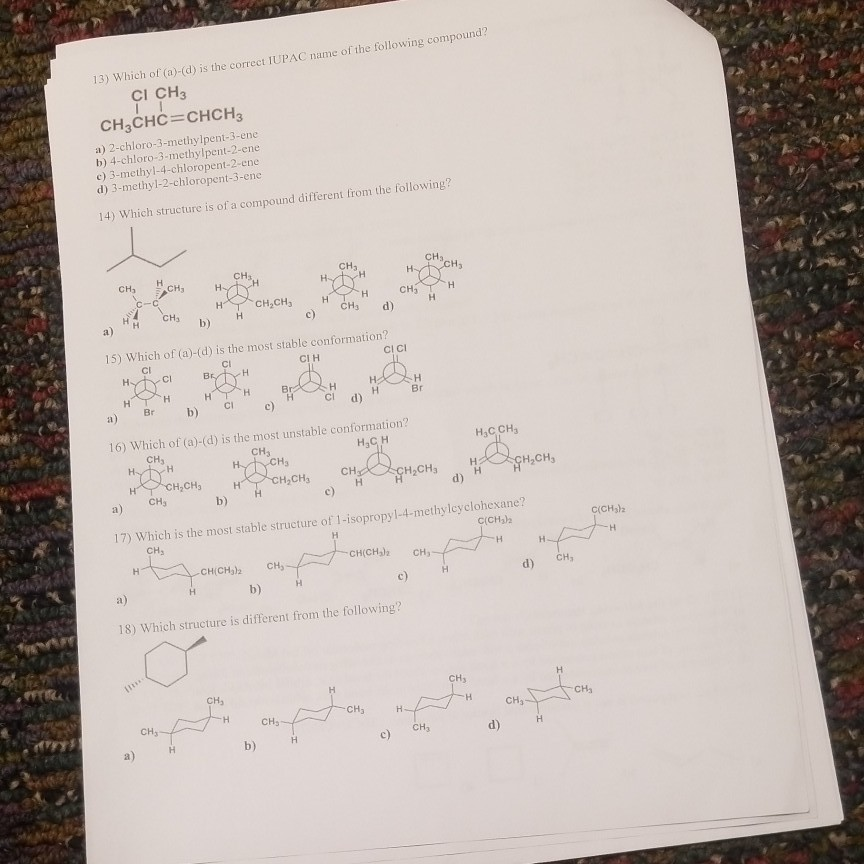



13 Which Of A D Is The Correct Iupac Name Of The Chegg Com



1




3r 2 Chloro 3 Methylpenta 1 4 Diene Structure C6h9cl Over 100 Million Chemical Compounds Mol Instincts




Solved 109 The Iupac Name Of The Compound Ch2ch3 Hzc A Trans 2 Chloro 3 Iodo Pent 2 Ene You Missed B Cis 2 Chloro 3 Iodo 2 Pent 2 Ene Trans 3 Indo 4 Chloro 3 Nentene



2 Chloro 3 Methyl Pentan 1 Ol Chemsink




Solved If 2 Chloro 3 Methyl Pentane Is Treated With Ethanolic Koh Solution How Many Different Alkenes Would Be Formed Via E2 Elimination Reaction




D Question 5 Provide The Iupac Name For The Compound Below Sec Butylchloromethylacetylen 5 Chloro 2 Methylpent 3 Yne 1 Chloro 4 4 Dimcthylbut 2 Homeworklib



Is 3 Methyl 2 Pentene Optically Active Quora



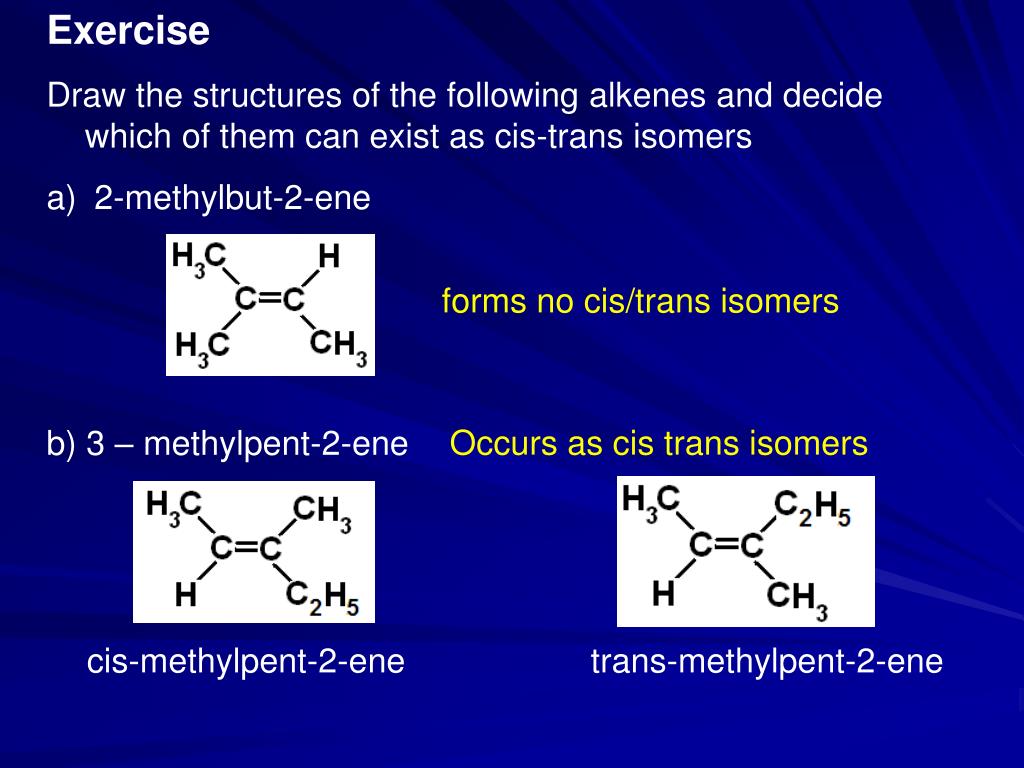
When Is The Cis Trans System Not Effective For Naming Geometric Isomers Socratic




Solution Organic Homework Studypool




11 Which One Would Be E Isomer A B Br Oh 12 Chegg Com




Write Down The Correct Mechanism For The Reaction Between 3 Methyl 2 Pentene And Hcl Study Com



Which Of The Following Compounds Are Capable Of Ciis And Trans Isomerism Why 4 Methylpent 2 Ene 3 Methylpent 1 Ene 4 Chlorohex 2 Ene Quora




E 2 3 Dibromo 4 Methylpent 2 Enoic Acid




11 Which One Would Be E Isomer A B Br Oh 12 Chegg Com



E 3 Chloro 2 Methyl Prop 2 En 1 Ol C4h7clo Chemspider




Draw The Structure S Of The Alkene S With The Molecular Formula C6h12 That Have A Single Methyl Homeworklib




Chemsheets As006 Electron Arrangement Ppt Video Online Download




File E 3 Methylpent 2 Ene 0 Svg Wikimedia Commons



1 Chloro 4 Methylpent 2 Ene Get Quote
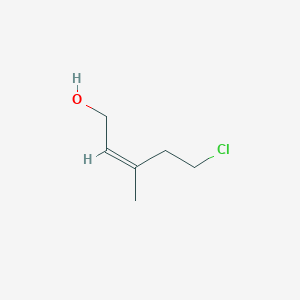



Z 5 Chloro 3 Methylpent 2 En 1 Ol C6h11clo Pubchem



Solved B Draw And Clearly Label Z And E Isomers For Any Of The 10 Compounds That Have Geometric Isomers Course Hero



1 Chloro 3 Methyl 2 Butene C5h9cl Chemspider



E 1 Chloro 3 Methyl Pent 2 Ene Chemsink




17 Edition Chemistry As C2 5 Organic Chemistry Ppt Download
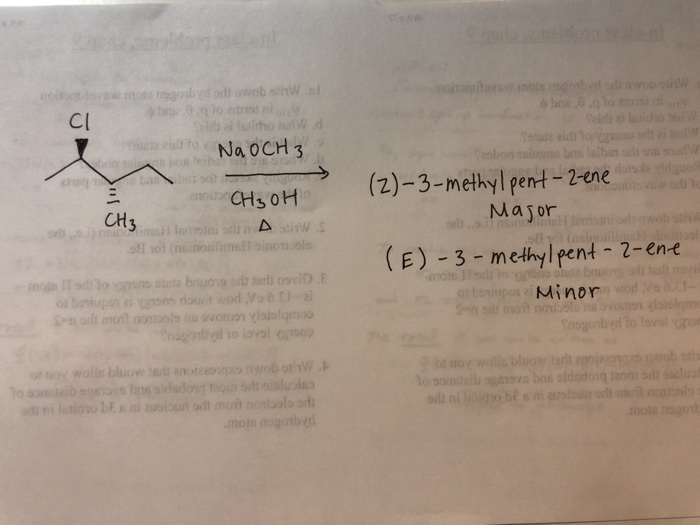



2r 3s 2 Chloro 3 Methylpentane Reacts With Sodium Chegg Com




Draw The Bond Line Structures Of The Following Compound Whose Iupac Names Are Given As Under A 2 Bromobutane B 1 Chloro 3 Methylbutane C 2 Bromo 2 Methylpropane D 4 Chloro 4 Methylpent 2 Ene E




1 Chloro 3 Methyl 2 Butene 95 503 60 6



But 1 Ene
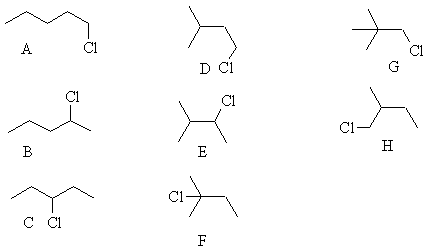



Ass 1aa3 Org



2e 5 Chloro 3 Methyl 2 Penten 1 Ol C6h11clo Chemspider



Final Exam Answer Key




Draw The Bond Line Structures Of The Following Compound Whose Iupac Names Are Given As Under A 2 Bromobutane B 1 Chloro 3 Methylbutane C 2 Bromo 2 Methylpropane D 4 Chloro 4 Methylpent 2 Ene E
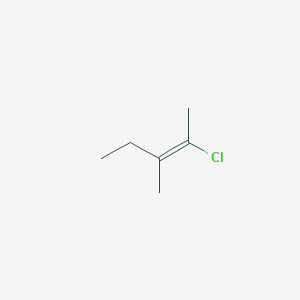



E 2 Chloro 3 Methylpent 2 Ene C6h11cl Pubchem
-2-bromo-3-methylbutane_small.png)



Organic Chemistry Alkenes




What Major E2 Product Would Form On The Reaction Of 2s 3r 2 Bromo 3 Methylpentane With Base Quesliui What Homeworklib




2e 2 Chloro 3 Methylpent 2 Ene Structure C6h11cl Over 100 Million Chemical Compounds Mol Instincts
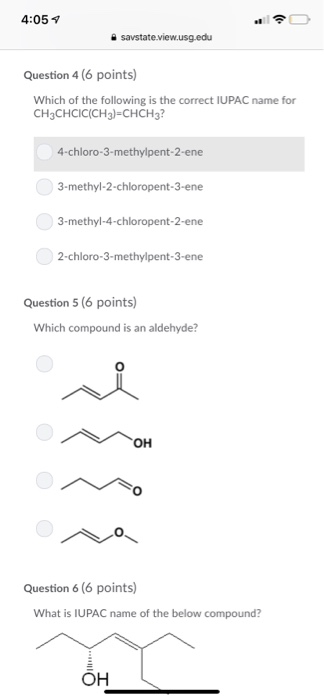



4 05 4 Savstate View Usg Edu Question 4 6 Points Chegg Com




3 Methyl 3 Penten 2 One Wikipedia



E 3 Chloro 4 Methyl Pent 2 Ene Chemsink




Alkyl Halides Elimination Reaction With 2r 3r 2 Chloro 3 Methylpentane Draw The Product Formed When 2r 3r 2 Chloro 3 Methylpentane Under Goes An Elimination Reaction Homeworklib
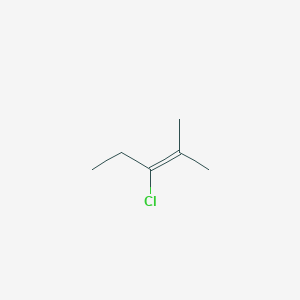



3 Chloro 2 Methylpent 2 Ene C6h11cl Pubchem



3 Chloro 2 Methyl 2 Pentene C6h11cl Chemspider



2



4 Chloro 3 Methylbut 2 En 1 Ol C5h9clo Chemspider




How To Draw The Structure For 3 Methylpent 1 Ene Drawing Alkenes Organic Chemistry Youtube



3 Chloro 2 Methyl Pent 1 Ene Chemsink




2e 2 Chloro 3 Methylpent 2 Ene Structure C6h11cl Over 100 Million Chemical Compounds Mol Instincts



Question 47fbe Socratic



Ppt Chemistry 3 5 Powerpoint Presentation Free Download Id




Solved 2 Chloro 2 Methylpentane On Reaction With Sodium Methoxide In Methanol Yields



4 Chloro 2 Methyl 2 Pentene C6h11cl Chemspider
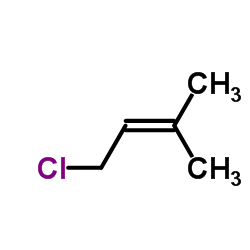



1 Chloro 3 Methyl 2 Butene Cas 503 60 6 Chemsrc
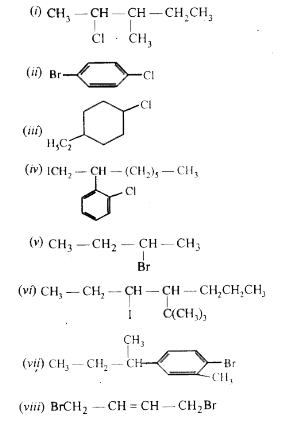



Write The Structures Of The Following Organic Halogen Compounds I 2 Chloro 3 Methylpentane Ii P Bromochlorobenzene Iii 1 Chloro 4 Ethylcyclohexane Iv 2 2 Chlorophenyl 1 Iodooctane V 2 Bromobutane Vi 4 Tert Butyl 3 Iodoheptane Vii




The Iupac Name Of The Following Compound Is




Solved Name The Following Molecules With The Appropriate Stereochemical Designation



Which Of The Following Is The Major Product In The Chegg Com




13 0 Alkenes Exam Q S Flashcards Quizlet



4 Chloro 2 Methylpent 2 Ene C6h11cl Pubchem




Solved The Iupac Name Of The Compound



Z 3 Methylpent 2 Ene



Structural




11 Which One Would Be E Isomer A Br B C D 12 Chegg Com



E 2 Chloro 3 Methyl Pent 2 Ene Chemsink




Draw The Bond Line Structures Of The Following Compound Whose Iupac Youtube




2 Chloro 4 Methyl Pent 2 Ene C6h11cl Pubchem




Identify All The Possible Alkenes That Would Be Formed On The D



2e 1 Bromo 3 Methylpent 2 Ene Get Quote




E 2 Cyanoperfluoro 3 Methylpent 2 Ene Spectrabase



Write Structures Of The Following Compounds I 2 Chloro 3 Methylpentane Sarthaks Econnect Largest Online Education Community



2 Chloro 3 Methyl 1 Pentene C6h11cl Chemspider




When 3 Methylpent 2 Ene Is Treated With Mercury Ii Acetate In Methanol And The Resulting Product Brainly Com



2e 2 Bromo 5 Chloro 3 Methylpent 2 Ene



1 Chemistry 1a6 01 02 Tutorial Problem Set 8



For The Molecule Chegg Com




Cis 3 Methyl 2 Pentene 97 0 Gc 922 62 3



0 件のコメント:
コメントを投稿